Comprehensive Solubility Rules Table Dataset
This dataset provides a detailed table of solubility rules for common ionic compounds, including their formulas, solubility status, exceptions, and illustrative examples. It's an essential resource for chemistry studies and research.
Free Download
Key Takeaways
- Access 31 key solubility rules for various ions and compounds.
- Explore detailed exceptions and notes for each solubility guideline.
- Download a ready-to-use reference for chemistry coursework and lab work.
- Get practical examples of soluble and insoluble compounds.
Showing 31 of 31
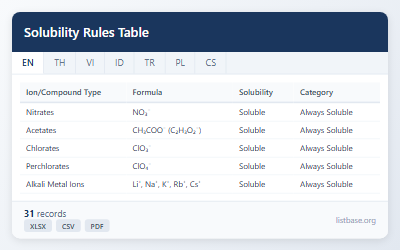
| Ion/Compound Type | Formula | Solubility | Exceptions/Notes | Category | Examples |
|---|---|---|---|---|---|
| Nitrates | NO₃⁻ | Soluble | None - all nitrates are soluble | Always Soluble | NaNO₃, KNO₃, AgNO₃, Pb(NO₃)₂ |
| Acetates | CH₃COO⁻ (C₂H₃O₂⁻) | Soluble | Silver acetate is slightly soluble | Always Soluble | NaCH₃COO, KC₂H₃O₂, Ca(CH₃COO)₂ |
| Chlorates | ClO₃⁻ | Soluble | None - all chlorates are soluble | Always Soluble | NaClO₃, KClO₃, Ba(ClO₃)₂ |
| Perchlorates | ClO₄⁻ | Soluble | KClO₄, RbClO₄, CsClO₄ are slightly soluble | Always Soluble | NaClO₄, Mg(ClO₄)₂, Ca(ClO₄)₂ |
| Alkali Metal Ions | Li⁺, Na⁺, K⁺, Rb⁺, Cs⁺ | Soluble | None - all Group 1 salts are soluble | Always Soluble | NaCl, KBr, Li₂SO₄, CsNO₃ |
| Ammonium | NH₄⁺ | Soluble | None - all ammonium salts are soluble | Always Soluble | NH₄Cl, NH₄NO₃, (NH₄)₂SO₄, NH₄OH |
| Halides (Cl⁻, Br⁻, I⁻) | Cl⁻, Br⁻, I⁻ | Mostly Soluble | Ag⁺, Pb²⁺, Hg₂²⁺ halides are insoluble | Soluble with Exceptions | NaCl, KBr soluble; AgCl, PbI₂ insoluble |
| Fluorides | F⁻ | Mostly Soluble | Group 2 fluorides (MgF₂, CaF₂, BaF₂) and PbF₂ are insoluble | Soluble with Exceptions | NaF, KF soluble; CaF₂, BaF₂ insoluble |
| Sulfates | SO₄²⁻ | Mostly Soluble | BaSO₄, PbSO₄, HgSO₄, CaSO₄, SrSO₄, Ag₂SO₄ are insoluble or slightly soluble | Soluble with Exceptions | Na₂SO₄, MgSO₄ soluble; BaSO₄, PbSO₄ insoluble |
| Carbonates | CO₃²⁻ | Mostly Insoluble | Alkali metal and ammonium carbonates are soluble | Insoluble with Exceptions | CaCO₃, BaCO₃ insoluble; Na₂CO₃, K₂CO₃ soluble |
| Phosphates | PO₄³⁻ | Mostly Insoluble | Alkali metal and ammonium phosphates are soluble | Insoluble with Exceptions | Ca₃(PO₄)₂, Ag₃PO₄ insoluble; Na₃PO₄ soluble |
| Chromates | CrO₄²⁻ | Mostly Insoluble | Alkali metal and ammonium chromates are soluble; MgCrO₄, CaCrO₄ are soluble | Insoluble with Exceptions | PbCrO₄, BaCrO₄, Ag₂CrO₄ insoluble; K₂CrO₄ soluble |
| Sulfides | S²⁻ | Mostly Insoluble | Alkali metal, alkaline earth, and ammonium sulfides are soluble | Insoluble with Exceptions | CuS, PbS, Ag₂S insoluble; Na₂S, (NH₄)₂S soluble |
| Hydroxides | OH⁻ | Mostly Insoluble | Alkali metal hydroxides are soluble; Ca(OH)₂, Sr(OH)₂, Ba(OH)₂ are slightly soluble | Insoluble with Exceptions | Fe(OH)₃, Al(OH)₃ insoluble; NaOH, KOH soluble |
| Oxides | O²⁻ | Mostly Insoluble | Alkali metal oxides react with water to form soluble hydroxides; CaO, SrO, BaO slightly soluble | Insoluble with Exceptions | Fe₂O₃, Al₂O₃ insoluble; Na₂O reacts with water |
| Sulfites | SO₃²⁻ | Mostly Insoluble | Alkali metal and ammonium sulfites are soluble | Insoluble with Exceptions | CaSO₃, BaSO₃ insoluble; Na₂SO₃ soluble |
| Oxalates | C₂O₄²⁻ | Mostly Insoluble | Alkali metal and ammonium oxalates are soluble | Insoluble with Exceptions | CaC₂O₄, BaC₂O₄ insoluble; Na₂C₂O₄ soluble |
| Silver Chloride | AgCl | Insoluble | Dissolves in NH₃ solution forming [Ag(NH₃)₂]⁺ | Common Precipitate | White precipitate, Ksp = 1.8 × 10⁻¹⁰ |
| Silver Bromide | AgBr | Insoluble | Less soluble than AgCl | Common Precipitate | Light yellow precipitate, Ksp = 5.0 × 10⁻¹³ |
| Silver Iodide | AgI | Insoluble | Least soluble silver halide | Common Precipitate | Yellow precipitate, Ksp = 8.3 × 10⁻¹⁷ |
| Lead(II) Chloride | PbCl₂ | Slightly Soluble | More soluble in hot water | Common Precipitate | White precipitate, Ksp = 1.7 × 10⁻⁵ |
| Lead(II) Iodide | PbI₂ | Insoluble | More soluble in hot water (golden rain demo) | Common Precipitate | Bright yellow precipitate, Ksp = 7.9 × 10⁻⁹ |
| Lead(II) Sulfate | PbSO₄ | Insoluble | Dissolves in concentrated H₂SO₄ | Common Precipitate | White precipitate, Ksp = 1.6 × 10⁻⁸ |
| Barium Sulfate | BaSO₄ | Insoluble | Very insoluble - used in medical imaging | Common Precipitate | White precipitate, Ksp = 1.1 × 10⁻¹⁰ |
| Calcium Carbonate | CaCO₃ | Insoluble | Dissolves in acid; more soluble in CO₂-rich water | Common Precipitate | White precipitate (limestone, chalk), Ksp = 3.4 × 10⁻⁹ |
| Calcium Sulfate | CaSO₄ | Slightly Soluble | Gypsum, plaster of Paris | Common Precipitate | White solid, Ksp = 2.4 × 10⁻⁵ |
| Iron(III) Hydroxide | Fe(OH)₃ | Insoluble | Dissolves in acid | Common Precipitate | Rust-brown precipitate, Ksp = 6.3 × 10⁻³⁸ |
| Copper(II) Hydroxide | Cu(OH)₂ | Insoluble | Dissolves in NH₃ forming [Cu(NH₃)₄]²⁺ (deep blue) | Common Precipitate | Blue precipitate, Ksp = 2.2 × 10⁻²⁰ |
| Magnesium Hydroxide | Mg(OH)₂ | Slightly Soluble | Milk of Magnesia - antacid | Common Precipitate | White precipitate, Ksp = 1.8 × 10⁻¹¹ |
| Aluminum Hydroxide | Al(OH)₃ | Insoluble | Amphoteric - dissolves in both acid and base | Common Precipitate | White gelatinous precipitate, Ksp = 3 × 10⁻³⁴ |
| Zinc Hydroxide | Zn(OH)₂ | Insoluble | Amphoteric - dissolves in excess NaOH | Common Precipitate | White precipitate, Ksp = 3 × 10⁻¹⁷ |
Use Cases
- Import the CSV file into your SQL database or Python script for programmatic analysis and integration into educational apps.
- Use the Excel file to filter, sort, and create custom reports for chemistry assignments or laboratory data analysis.
- Print the PDF version for a handy, offline reference chart in classrooms or during lab experiments.
- Leverage this dataset to quickly verify the solubility of various compounds for chemical reaction predictions and experimental design.